I started my work on the relationships between the structure of inorganic historical pigments and their production methods during my PhD (dir. M. Menu, co-supervised by G. Wallez and T. Calligaro). We were interested in the lead white pigment, omnipresent in paintings from the Antiquity till the 20th c. Lead white is a mixture of lead carbonates with varying degrees of hydration, cerussite PbCO3 and hydrocerussite Pb3(CO3)2.(OH)2, of synthetic origin (obtained by corrosion of metallic lead under acetic acid vapors). The stability of the two phases depends on pH (cerussite is stable at pH < 6 and hydrocerussite at 8 < pH < 10). We clarified the mechanisms involved in this corrosion, and demonstrated that variations in pigment synthesis conditions (O2 and CO2 levels, temperature, corrosion time), as well as the use of post-synthesis processes (in particular treatment in acidic environments, heating in aqueous environments) had a significant influence on two structural parameters: the hydrocerussite:cerussite ratio and the morphology of the crystallites [1]. Quantifying these parameters in paints therefore provides valuable information for reconstructing ancient manufacturing methods.
We conducted a structural study on an extensive artistic corpus, using different XRD analysis configurations (laboratory prototype [2], synchrotron radiation [3]) coupled with the Rietveld refinement method: we were able to precisely quantify the crystalline phases of lead white, model the pigment crystallites at the nanometric scale, and obtain the distribution of phases at the micro-scale in historical micro-samples [4]. By comparing these results with those obtained on model samples, we were able to identify the post-synthesis processes applied to lead carbonate pigments used in the Italian Renaissance.

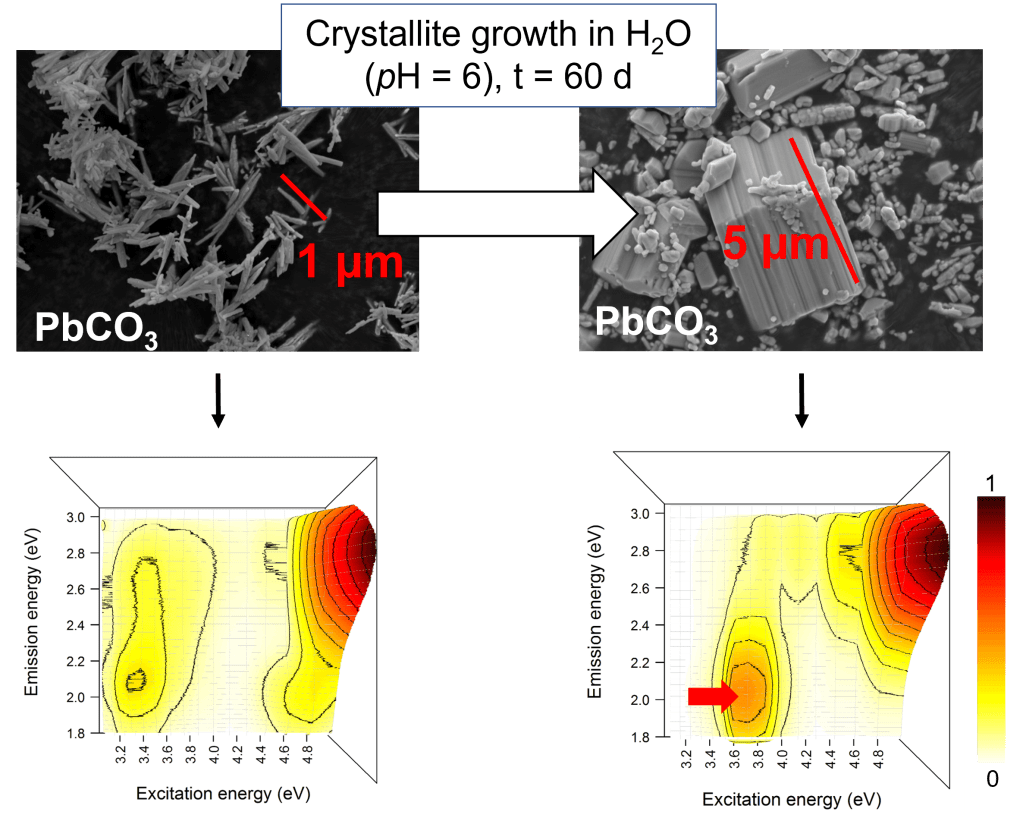
We also studied the photoluminescence (PL) properties of the constitutive phases of lead white in order to identify potential signatures of their synthesis and their use in paintings. We assigned the PL bands in the visible range to charge transfer transitions of the type Pb3+-CO33− → Pb2+(1S0)-CO32− + hν and showed that at high excitation energy (5 eV, 250 nm), the excitation of the carbonate matrix induces different PL emission bands for the two constituent phases of the pigment (cerussite at 2.8 eV and hydrocerussite at 2.1 eV). We therefore proposed the use of PL spectroscopy to discriminate between these crystalline compounds in paints.
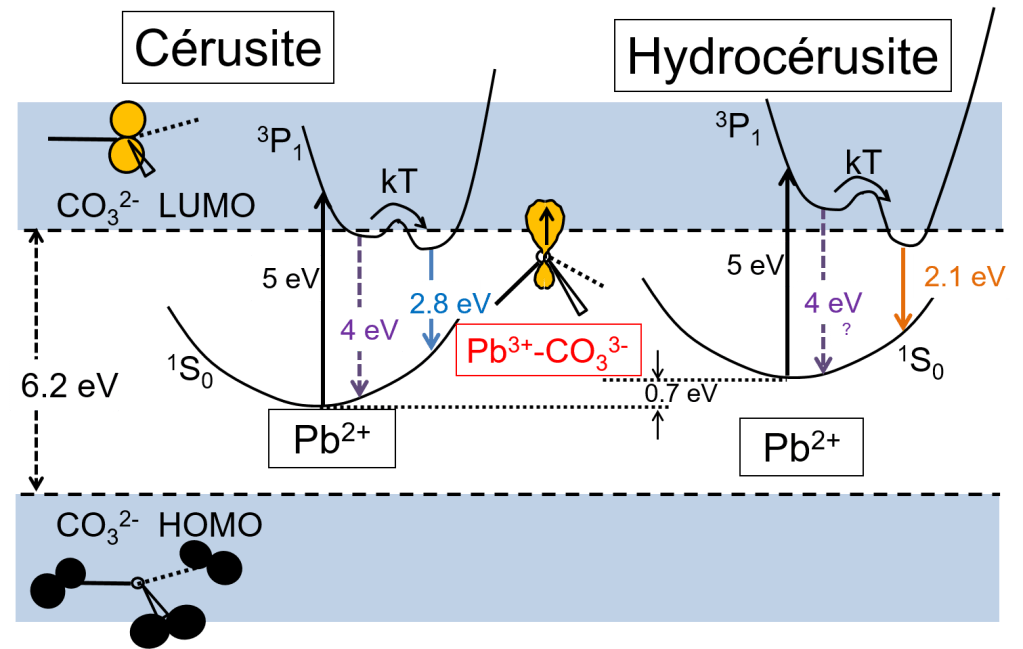
Finally, we evaluated the potential of PL properties to provide markers for the synthesis of ancient pigments. Two polymorphs of a pigment that are indistinguishable by structural analysis, obtained by different synthesis methods, differ in the presence of structural defects or impurities, which result in variations in the PL signal. In the case of lead carbonates, we have shown that PL emissions excited below 4.5 eV were attributed to the charge transfer transitions, disturbed by defects present in the cerussite and hydrocerussite matrices. We then shown that the application of post-synthesis treatments affected PL emissions excited in the near UV, proving that PL spectroscopy can be considered an innovative probe for studying the origin and evolution of ancient pictorial materials [5].